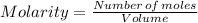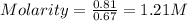Chemistry, 06.12.2019 23:31 muravyevaarina
A0.67 l solution of ammonium sulfate, (nh4)so4, contains 0.81 mole of the solute. what is the approximate molarité of the solution

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 23.06.2019 00:00
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1

Chemistry, 23.06.2019 01:30
What happens to the concentration of hydronium ions as the ph of a solution increases? a. hydronium ion concentration stays the same b. hydronium ion concentration decreases c. hydronium ion concentration increases
Answers: 1
You know the right answer?
A0.67 l solution of ammonium sulfate, (nh4)so4, contains 0.81 mole of the solute. what is the approx...
Questions



Biology, 20.10.2020 23:01

Biology, 20.10.2020 23:01


History, 20.10.2020 23:01


Health, 20.10.2020 23:01

Mathematics, 20.10.2020 23:01

History, 20.10.2020 23:01





Mathematics, 20.10.2020 23:01

Arts, 20.10.2020 23:01