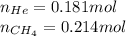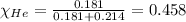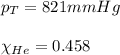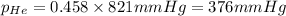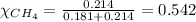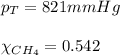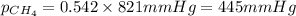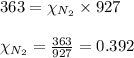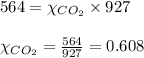Amixture of helium and methane gases, at a total pressure of 821 mm hg, contains 0.723 grams of helium and 3.43 grams of methane. what is the partial pressure of each gas in the mixture?
phe = mm hg
pch4 = mm hg
2.) a mixture of nitrogen and carbon dioxide gases contains nitrogen at a partial pressure of 363 mm hg and carbon dioxide at a partial pressure of 564 mm hg. what is the mole fraction of each gas in the mixture?
xn2 =
xco2 =

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3
You know the right answer?
Amixture of helium and methane gases, at a total pressure of 821 mm hg, contains 0.723 grams of heli...
Questions

Mathematics, 21.11.2020 14:00



Advanced Placement (AP), 21.11.2020 14:00

Mathematics, 21.11.2020 14:00



Mathematics, 21.11.2020 14:00

Chemistry, 21.11.2020 14:00


Mathematics, 21.11.2020 14:00

Mathematics, 21.11.2020 14:00


Physics, 21.11.2020 14:00


Mathematics, 21.11.2020 14:00

English, 21.11.2020 14:00


Mathematics, 21.11.2020 14:00

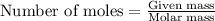 .....(1)
.....(1)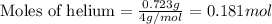
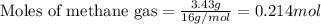
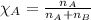 .......(2)
.......(2)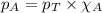 ......(3)
......(3)