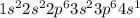Chemistry, 06.12.2019 20:31 angelkiss2019
How does the electron configuration of elements within the same group compare? (a.) they all have their valence electrons in the same energy level. ( b) they all have their valence electrons in the same type of subshell. (c) they all have their valence electrons in a p suborbital. ( d) they all have their valence electrons in the 3rd energy level.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2
You know the right answer?
How does the electron configuration of elements within the same group compare? (a.) they all have t...
Questions



Social Studies, 10.10.2019 22:50


History, 10.10.2019 22:50



Mathematics, 10.10.2019 22:50



Mathematics, 10.10.2019 22:50


English, 10.10.2019 22:50


History, 10.10.2019 22:50

History, 10.10.2019 22:50


History, 10.10.2019 22:50

Mathematics, 10.10.2019 22:50

Mathematics, 10.10.2019 22:50

 ' orbital and because they are in the first column they all have
' orbital and because they are in the first column they all have 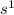 configuration. i.e,H
configuration. i.e,H 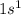 Li
Li 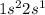 Na
Na 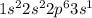 K
K