
Chemistry, 06.12.2019 19:31 alyssalefeber
The molal boiling point elevation constant kb= 2.13 ℃kgmo-for a certain substance x, when 12. g of urea are dissolved in 100. g of x, the solution boils at 126.3 °c. calculate the boiling point of pure x. be sure your answer has the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2


Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2
You know the right answer?
The molal boiling point elevation constant kb= 2.13 ℃kgmo-for a certain substance x, when 12. g of u...
Questions

Biology, 21.05.2021 17:00



Mathematics, 21.05.2021 17:00

Mathematics, 21.05.2021 17:00

Mathematics, 21.05.2021 17:00


Mathematics, 21.05.2021 17:00




Mathematics, 21.05.2021 17:00

Mathematics, 21.05.2021 17:00


Mathematics, 21.05.2021 17:00





Chemistry, 21.05.2021 17:00

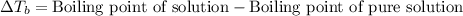
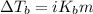
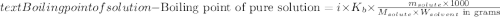
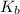 = molal boiling point elevation constant = 2.13°C/m
= molal boiling point elevation constant = 2.13°C/m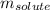 = Given mass of solute (urea) = 12. g
= Given mass of solute (urea) = 12. g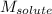 = Molar mass of solute (urea) = 60 g/mol
= Molar mass of solute (urea) = 60 g/mol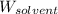 = Mass of solvent (X) = 100. g
= Mass of solvent (X) = 100. g


