
Chemistry, 06.12.2019 19:31 anthonyvargas8780
Astandard nitric acid solution is prepared using 0.425 g of sodium carbonate, na2co3. the balanced equation is:
2 hno3 (aq) + na2co3 (s) ➝ 2 nano3 (aq) + h2o (l) + co2 (g)
referring to example exercise 2, provide in three significant figures:
a) # moles of na2co3
b) # moles of hno3
c) molarity of hno3 if 33.25 ml are required to reach a permanent endpoint.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
How many atoms of oxygen are contained in 160 grams of n2o3
Answers: 2

Chemistry, 22.06.2019 07:40
The formation of a solid, also known as a is an indication of a chemical change. precipitate particulate particle powder
Answers: 3

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2
You know the right answer?
Astandard nitric acid solution is prepared using 0.425 g of sodium carbonate, na2co3. the balanced e...
Questions


Mathematics, 01.03.2021 23:10



Advanced Placement (AP), 01.03.2021 23:10

English, 01.03.2021 23:10


English, 01.03.2021 23:10



Mathematics, 01.03.2021 23:10


Biology, 01.03.2021 23:10

Social Studies, 01.03.2021 23:10

Mathematics, 01.03.2021 23:10


English, 01.03.2021 23:10

Mathematics, 01.03.2021 23:10


Mathematics, 01.03.2021 23:10

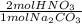 = 8.02x10⁻³ mol HNO₃
= 8.02x10⁻³ mol HNO₃

