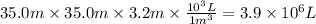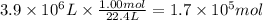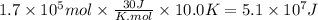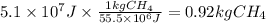Chemistry, 06.12.2019 17:31 keshewar2671
Assuming that all the energy given off in the reaction goes to heating up only the air in the house, determine the mass of methane required to heat the air in a house by 10.0 ∘c. assume each of the following: house dimensions are 35.0 m × 35.0 m × 3.2 m ; specific heat capacity of air is 30 j/k⋅mol; 1.00 mol of air occupies 22.4l for all temperatures concerned.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2


Chemistry, 22.06.2019 20:50
What is the vapor pressure of a solution with a benzene to octane?
Answers: 2
You know the right answer?
Assuming that all the energy given off in the reaction goes to heating up only the air in the house,...
Questions


Mathematics, 20.09.2019 16:00



Mathematics, 20.09.2019 16:00

Mathematics, 20.09.2019 16:00


Social Studies, 20.09.2019 16:00

Social Studies, 20.09.2019 16:00





Mathematics, 20.09.2019 16:00

Mathematics, 20.09.2019 16:00

Mathematics, 20.09.2019 16:00



Social Studies, 20.09.2019 16:00

Computers and Technology, 20.09.2019 16:00