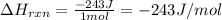4). one mole of monoclinic sulfur at 25c was placed in a constant-pressure calorimeter whose heat capacity (c) was 1620 j/k. the temperature of the calorimeter increased by 0.150 co when the sulfur changed from the monoclinic to the orthorhombic form. calculate the enthalpy change for the process s(monoclinic) s(orthorhombic).

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
About 70 percent of the earth's surface is water-covered, and about 96.5 percent of all earth's water is salt water. identify the watery feature on earth that is made of freshwater rather than salt water. a) bay b) glacier c) ocean d) sea it is not incomplete this is the true question
Answers: 1

Chemistry, 22.06.2019 04:10
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1
You know the right answer?
4). one mole of monoclinic sulfur at 25c was placed in a constant-pressure calorimeter whose heat c...
Questions


Social Studies, 17.02.2021 20:50

Mathematics, 17.02.2021 20:50

English, 17.02.2021 20:50


Mathematics, 17.02.2021 20:50

Mathematics, 17.02.2021 20:50

Mathematics, 17.02.2021 20:50


Arts, 17.02.2021 20:50

Mathematics, 17.02.2021 20:50

History, 17.02.2021 20:50



Business, 17.02.2021 20:50

Mathematics, 17.02.2021 20:50



Computers and Technology, 17.02.2021 20:50

Chemistry, 17.02.2021 20:50

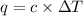
 = change in temperature =
= change in temperature = 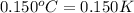 (Change remains same)
(Change remains same)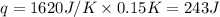
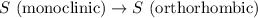
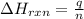
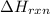 = enthalpy change of the reaction
= enthalpy change of the reaction