

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1

Chemistry, 22.06.2019 00:00
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3

Chemistry, 22.06.2019 07:00
Which set of characteristics best describes igneous rock? a) largest type of rock, made of organic matter, hardest type of rock b) least abundant type of rock, made of other rocks, made mostly of minerals c) found on all continents, contains wavy bands of stripes, contains fossils d) most abundant type in earth's crust, made of magma/lava, contains no fossils
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1
You know the right answer?
Asample of hydrogen gas has an initial pressure of 2.86 atm and an initial volume of 8472 ml if the...
Questions

Social Studies, 07.01.2021 21:20

Mathematics, 07.01.2021 21:20


Mathematics, 07.01.2021 21:20

Mathematics, 07.01.2021 21:20

Physics, 07.01.2021 21:20

Mathematics, 07.01.2021 21:20



Mathematics, 07.01.2021 21:20

Mathematics, 07.01.2021 21:20

Business, 07.01.2021 21:20

Biology, 07.01.2021 21:20

Mathematics, 07.01.2021 21:20

Physics, 07.01.2021 21:20

Mathematics, 07.01.2021 21:20




Mathematics, 07.01.2021 21:20

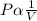 This means , PV = k (constant)Therefore; P₁V₁ = P₂V₂Rearranging the formula, we can get the new pressure, P₂
This means , PV = k (constant)Therefore; P₁V₁ = P₂V₂Rearranging the formula, we can get the new pressure, P₂

