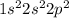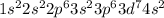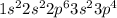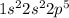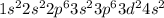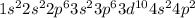Chemistry, 06.12.2019 06:31 anicholson41
According to hund's rule of maximum spin multiplicity, how many singly-occupied orbitals are there in the valence shells of the following elements in their ground states? enter your answer as the sum of all the orbitals (for example 15).
a) carbon
b) cobalt
c) sulfur
d) fluorine
e) titanium
f) germanium

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1
You know the right answer?
According to hund's rule of maximum spin multiplicity, how many singly-occupied orbitals are there i...
Questions







Mathematics, 16.07.2019 01:00

Mathematics, 16.07.2019 01:00






Physics, 16.07.2019 01:00


History, 16.07.2019 01:00



History, 16.07.2019 01:00

Mathematics, 16.07.2019 01:00