
Aresearcher used hplc to examine a bicomponent mixture containing 1.22 mg/l of compound a and 1.31 mg/l of compound b, which was added as an internal standard. this mixture produced peak areas for compounds a and b of 10919 and 5379 , respectively. using the above information, determine the response factor (f).
after establishing f, the researcher prepared a solution by combining 8.18 mg of b with 10.00 ml of an unknown solution containing only a and then diluted it to a final volume of 50.00 ml. the sample was examined using hplc and peak areas of 6065 and 9111 were observed for a and b, respectively.
determine the concentration of a (mg/ml) in the unknown solution.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1

Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1

Chemistry, 22.06.2019 23:10
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3
You know the right answer?
Aresearcher used hplc to examine a bicomponent mixture containing 1.22 mg/l of compound a and 1.31 m...
Questions






Spanish, 26.10.2019 06:43

Social Studies, 26.10.2019 06:43







English, 26.10.2019 06:43


History, 26.10.2019 06:43

Biology, 26.10.2019 06:43



Advanced Placement (AP), 26.10.2019 06:43

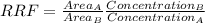
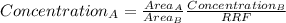
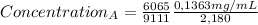
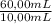 = 02497 mg/mL
= 02497 mg/mL

