

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 22:30
What if it is did darwin used to support his theory of evolution
Answers: 1

Chemistry, 22.06.2019 23:50
Which scientists contributed to the determination of how cfcs in clouds in the upper atmosphere could destroy ozone molecules
Answers: 1
You know the right answer?
When nh3(g) reacts with o2(g), the products of the combustion are no(g) and h2o(g). what volume of o...
Questions

English, 04.03.2021 04:00


Mathematics, 04.03.2021 04:00


Mathematics, 04.03.2021 04:00


Mathematics, 04.03.2021 04:00



Mathematics, 04.03.2021 04:00




English, 04.03.2021 04:00

Mathematics, 04.03.2021 04:00

Computers and Technology, 04.03.2021 04:00




Mathematics, 04.03.2021 04:00

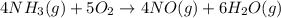
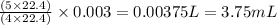 of oxygen gas
of oxygen gas

