Chemistry, 06.12.2019 05:31 AaronMicrosoft15
If the equilibrium constant for a two-electron redox reaction at 298 k is 1.0×10−4, calculate the corresponding δg∘ and e∘cel under standard conditions.
δg∘ =
e∘cell =

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:30
Asap , 8th grade science 50 points 1) a 2kg bowling ball sits on top of a building that is 40 m tall. what type of energy does it have? potential energy energy of acceleration kinetic energy chemical energy 2) a 2 kg bowling ball sits on top of a building that is 40 meters tall. how much potential energy does it have? 784 j 80 j 784 n 78.4 j 3) a 4 kg bowling ball rolls at a speed of 5 m/s on the roof of a building that is 30 meters tall. what type of energy does the bowling ball have? both potential and kinetic energy kinetic energy potential energy speed 4) a 4 kg bowling ball rolls at a speed of 5 m/s on the roof of a building that is 30 meters tall. what is its kinetic energy? 50 j 10 j 120 j 1,176 j 5) an 8 kg bowling ball rolls at a speed of 10 m/s on the ground. what type of energy does the bowling ball have? kinetic acceleration potential speed 6) an 8 kg bowling ball rolls at a speed of 10 m/s on the ground. what is its kinetic energy? 40 j 80 j 160 j 400 j 7) a 20 kg child is tossed in the air by her parent. what is her kinetic energy when she is 2 meters off the ground traveling at 5 m/s? 200 j 40 j 100 j 250 j 8) a 1,000 kg car has 50,000 j of kinetic energy. what is its speed? 100 m/s 150 m/s 50 m/s 10 m/s 9) a 200 kg boulder has 39,200 j of gravitational potential energy. what height is it at? 50 m 20 m 150 m 200 m 10) a 1 kg model airplane has 12.5 j of kinetic energy and 98 j of gravitational potential energy. what is its speed? 5 m/s 10 m/s 15 m/s 20 m/s
Answers: 1

Chemistry, 22.06.2019 04:50
The name of the ion, s2-, is: sulfurous ion sulfide ion sulfur ion sulfate ion
Answers: 1

Chemistry, 22.06.2019 13:40
Can someone me with 6 to 10 plz this is for masteries test.
Answers: 1

Chemistry, 22.06.2019 14:00
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3
You know the right answer?
If the equilibrium constant for a two-electron redox reaction at 298 k is 1.0×10−4, calculate the co...
Questions


History, 01.07.2021 19:20


Biology, 01.07.2021 19:20

Chemistry, 01.07.2021 19:20

Chemistry, 01.07.2021 19:20


Mathematics, 01.07.2021 19:20




Mathematics, 01.07.2021 19:20



Biology, 01.07.2021 19:20


Mathematics, 01.07.2021 19:20


Mathematics, 01.07.2021 19:20

History, 01.07.2021 19:20

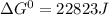
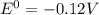
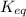
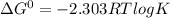
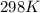
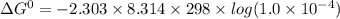
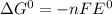
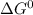 = gibbs free energy = 22823J
= gibbs free energy = 22823J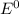 = standard emf
= standard emf