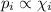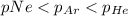Chemistry, 06.12.2019 05:31 zachcochran2007
Amixture of he, ne, and ar gases at a particular temperature has partial pressures of 0.75 atm he, 0.30 atm ne, and 0.62 atm ar. which of the following shows the correct trend for the mole fractions of the component gases?
a. latex: \chi_{ne}< \chi_{ar}< \chi_{he}b. latex: \chi_{ar}< \chi_{ne}< \chi_{he}c. latex: \chi_{he}< \chi_{ar}< \chi_{ne}d. latex: \chi_{he}< \chi_{ne}< \chi_{ar}

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 2

Chemistry, 22.06.2019 02:10
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1

Chemistry, 23.06.2019 06:00
Which of the following is a solution a- brewed coffee b-tomato juice c- ranch salad dressing d- muddy water
Answers: 1
You know the right answer?
Amixture of he, ne, and ar gases at a particular temperature has partial pressures of 0.75 atm he, 0...
Questions

Spanish, 31.10.2020 01:00

Mathematics, 31.10.2020 01:00


Mathematics, 31.10.2020 01:00

English, 31.10.2020 01:00

Chemistry, 31.10.2020 01:00

English, 31.10.2020 01:00

Mathematics, 31.10.2020 01:00

Physics, 31.10.2020 01:00



Social Studies, 31.10.2020 01:00

Mathematics, 31.10.2020 01:00







History, 31.10.2020 01:00

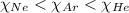
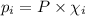
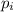 = partial pressure of the i component
= partial pressure of the i component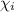 = Mole fraction of the i component
= Mole fraction of the i component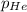 = 0.75 atm
= 0.75 atm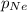 = 0.30 atm
= 0.30 atm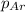 = 0.62 atm
= 0.62 atm