
Chemistry, 06.12.2019 05:31 DisneyyKayy
Calculate δh∘ in kilojoules for the reaction of acetylene (c2h2) (δh∘f=227.4kj/mol) with o2 to yield carbon dioxide (co2) (δh∘f=−393.5 kj/mol) and h2o(g) (δh∘f=−241.8kj/mol), a reaction which is supplied by the industrial gases industry for oxyacetylene gas welding and cutting due to the high temperature of the flame.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1

Chemistry, 23.06.2019 09:00
How many grams of ammonia are produced when 1.0 mole of nitrogen reacts
Answers: 2
You know the right answer?
Calculate δh∘ in kilojoules for the reaction of acetylene (c2h2) (δh∘f=227.4kj/mol) with o2 to yield...
Questions

English, 25.08.2019 08:50

Mathematics, 25.08.2019 08:50


Social Studies, 25.08.2019 08:50

Mathematics, 25.08.2019 08:50

Business, 25.08.2019 08:50


Mathematics, 25.08.2019 08:50


History, 25.08.2019 08:50

Chemistry, 25.08.2019 08:50

Mathematics, 25.08.2019 08:50

History, 25.08.2019 08:50







Mathematics, 25.08.2019 09:00

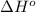 for the reaction is, -2512.4 kJ
for the reaction is, -2512.4 kJ
![\Delta H^o_{rxn}=[(3\times \Delta H^o_f_{(CO_2(g))})+(4\times \Delta H^o_f_{(H_2O(g))})]-[(1\times \Delta H^o_f_{(C_2H_2(g))})+(5\times \Delta H^o_f_{(O_2(g))})]](/tpl/images/0405/9362/62f19.png)
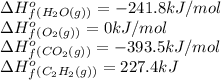
![\Delta H^o_{rxn}=[(4\times (-393.5))+(2\times (-241.8))]-[(2\times (227.4)+(5\times (0))]\\\\\Delta H^o_{rxn}=-2512.4kJ](/tpl/images/0405/9362/361f8.png)


