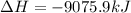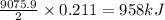Chemistry, 06.12.2019 05:31 lindseylewis313
B5h9(l) is a colorless liquid that will explode when exposed to oxygen. how much heat is released when 0.211 mol of b5h9 reacts with excess oxygen where the products are b2h3(s) and h2o(l). the standard enthalpy of formation of b5h9(l) is 73.2 kj/mol, the standard enthalpy of formation of b2h3(s) is -1272 kj/mol and that of h2o(l) is -285.4 kj/mol. express your answer in kj.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3

Chemistry, 22.06.2019 19:40
What causes different colors to appear in the sky? the absorption of light by air molecules the reflection of light by bodies of water the greenhouse effect in earth's atmosphere the scattering and reflection of light by dust particles
Answers: 2
You know the right answer?
B5h9(l) is a colorless liquid that will explode when exposed to oxygen. how much heat is released wh...
Questions


Computers and Technology, 25.04.2020 04:04


Mathematics, 25.04.2020 04:04

Chemistry, 25.04.2020 04:04


History, 25.04.2020 04:04



Mathematics, 25.04.2020 04:04






Biology, 25.04.2020 04:04


History, 25.04.2020 04:04


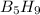 reacts with excess oxygen.
reacts with excess oxygen.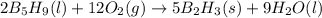
![\Delta H=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/0405/9477/76c37.png)
![\Delta H=[(n_{B_2H_3}\times \Delta H_{B_2H_3})+(n_{H_2O}\times \Delta H_{H_2O})]-[(n_{O_2}\times \Delta H_{O_2})+(n_{B_5H_9}\times \Delta H_{B_5H_9})]](/tpl/images/0405/9477/bc69c.png)
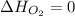 (as heat of formation of substances in their standard state is zero
(as heat of formation of substances in their standard state is zero![\Delta H=[(5\times -1272)+(9\times -285.5]-[(12\times 0)+(2\times 73.2)]](/tpl/images/0405/9477/56397.png)