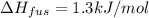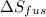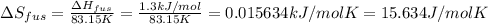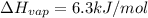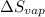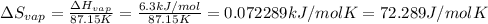Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1

Chemistry, 22.06.2019 23:00
What element has similar physical and chemical properties as boron.
Answers: 1

Chemistry, 22.06.2019 23:00
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1

Chemistry, 23.06.2019 01:00
The time that is taken by neptune once around the sun is called
Answers: 1
You know the right answer?
The molar heats of fusion and vaporization of argon are 1.3 kj/mol and 6.3 kj/mol respectively, and...
Questions

Mathematics, 07.08.2019 20:10






Chemistry, 07.08.2019 20:10









SAT, 07.08.2019 20:10



History, 07.08.2019 20:10