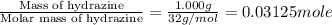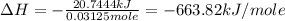Chemistry, 06.12.2019 03:31 isabeltorres5
A1.000 gram sample of the rocket fuel hydrazine (n2h4) is burned in a bomb calorimeter. the temperature rises from 24.62°c to 28.16°c. the heat capacity of the calorimeter (including the water) is 5860 j/°c. calculate the molar heat of combustion of hydrazine, in kj/mole.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2


Chemistry, 22.06.2019 22:30
What methods could you use to solubilize calcium carbonate
Answers: 1

Chemistry, 23.06.2019 05:30
The image compares the arrangement of electrons in two different neutral atoms. a figure labeled atom q has a shaded sphere at the center of three concentric circles. the innermost circle has two black spheres. the middle circle has six black spheres. to the left of this figure is another figure labeled atom p. atom p has a shaded sphere at the center of three concentric circles. the innermost circle has two black spheres. the middle circle has seven black spheres. which of the following best explains the position of the two atoms in the periodic table? atom p has an estimated zeff of 7 and is therefore to the left of atom q, which has a zeff of 6. atom p has an estimated zeff of 7 and is therefore to the right of atom q, which has a zeff of 6. atom p has an estimated zeff of 5 and is therefore below atom q, which has a zeff of 4. atom p has an estimated zeff of 5 and is therefore above atom q, which has a zeff of 4.
Answers: 3
You know the right answer?
A1.000 gram sample of the rocket fuel hydrazine (n2h4) is burned in a bomb calorimeter. the temperat...
Questions


Law, 09.02.2021 16:40



Advanced Placement (AP), 09.02.2021 16:40

Mathematics, 09.02.2021 16:40


History, 09.02.2021 16:40


Mathematics, 09.02.2021 16:50


Arts, 09.02.2021 16:50


Computers and Technology, 09.02.2021 16:50





Mathematics, 09.02.2021 16:50

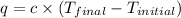
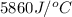
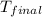 = final temperature =
= final temperature = 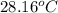
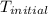 = initial temperature =
= initial temperature = 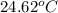
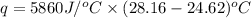
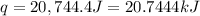
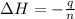
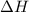 = enthalpy change = ?
= enthalpy change = ?