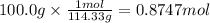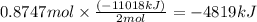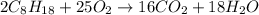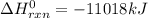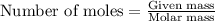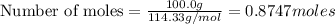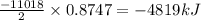Chemistry, 06.12.2019 02:31 autumnguidry7628
Using the following equation for the combustion of octane, calculate the heat associated with the combustion of 100.0 g of octane assuming complete combustion. the molar mass of octane is 114.33 g/mole. the molar mass of oxygen is 31.9988 g/mole.
2 c8h18 + 25 o2 → 16 co2 + 18 h2o δh°rxn = -11018 kj
a) -535.4 kj
b) -4819 kj
c) -602.3 kj
d) -385.5 kj
e) -11018 kj

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which is a character of nuclear fusion but not nuclear fission
Answers: 3



Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1
You know the right answer?
Using the following equation for the combustion of octane, calculate the heat associated with the co...
Questions

Biology, 03.11.2019 07:31

Mathematics, 03.11.2019 07:31


Chemistry, 03.11.2019 07:31

Mathematics, 03.11.2019 07:31



Mathematics, 03.11.2019 07:31

Social Studies, 03.11.2019 07:31

English, 03.11.2019 07:31




Mathematics, 03.11.2019 07:31



Biology, 03.11.2019 07:31

Chemistry, 03.11.2019 07:31