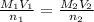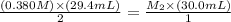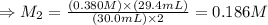Chemistry, 06.12.2019 02:31 NathanFrase6770
The amount of i − 3 ( aq ) in a solution can be determined by titration with a solution containing a known concentration of s 2 o 2 − 3 ( aq ) (thiosulfate ion). the determination is based on the net ionic equation 2 s 2 o 2 − 3 ( aq ) + i − 3 ( aq ) ⟶ s 4 o 2 − 6 ( aq ) + 3 i − ( aq ) given that it requires 29.4 ml of 0.380 m na 2 s 2 o 3 ( aq ) to titrate a 30.0 ml sample of i − 3 ( aq ) , calculate the molarity of i − 3 ( aq ) in the solution.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 21:50
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1

Chemistry, 23.06.2019 00:30
What is the percent by mass of magnesium sulfate in mgso4.7h2o
Answers: 3

Chemistry, 23.06.2019 13:30
Consider this reaction taking place in a closed 2 liter container: 2so2(g) + o2(g) → 2so3(g) if the volume of the container is decreased to 1 liter, what will happen to the equilibrium of the reaction? it will shift left. it will shift right. it will remain constant it will decrease by half
Answers: 3
You know the right answer?
The amount of i − 3 ( aq ) in a solution can be determined by titration with a solution containing a...
Questions

Mathematics, 23.11.2021 15:20

Mathematics, 23.11.2021 15:20

Computers and Technology, 23.11.2021 15:20



Spanish, 23.11.2021 15:20




World Languages, 23.11.2021 15:20



Mathematics, 23.11.2021 15:20


Chemistry, 23.11.2021 15:20

Mathematics, 23.11.2021 15:20


Social Studies, 23.11.2021 15:20

Social Studies, 23.11.2021 15:20

Social Studies, 23.11.2021 15:20