

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Will mark brainliest 26. which of these statements are true? (3 points) a. gases are compressible b. gases fill their containers completely c. the pressure of a gas is independent of the temperature d. gases have mass e. gases exert pressure f. the pressure of a gas is dependent on the volume g. gas pressure results from the collisions between gas particles h. gases have a definite volume and shape
Answers: 1

Chemistry, 21.06.2019 22:50
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 22.06.2019 01:30
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2

Chemistry, 22.06.2019 23:00
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2
You know the right answer?
16.34 g of cuso4 dissolved in water giving out 55.51 kj and 25.17 g cuso4•5h2o absorbs 95.31 kj. fro...
Questions




Mathematics, 11.03.2020 23:16

Mathematics, 11.03.2020 23:16




Biology, 11.03.2020 23:16






Mathematics, 11.03.2020 23:16





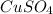 dissolved
dissolved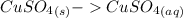 (Eq. 1)
(Eq. 1)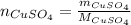
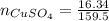
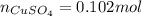
 dissolved
dissolved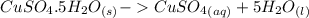 (Eq. 2)
(Eq. 2)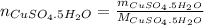


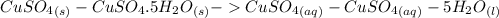 ΔH = -541.85-944.77
ΔH = -541.85-944.77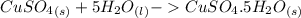 ΔH = -1486.62 kJ/mol
ΔH = -1486.62 kJ/mol

