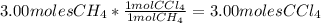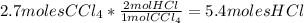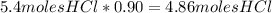Chemistry, 06.12.2019 00:31 babowmanjacob666
Each step in the following process has a yield of 90.0 %. ch 4 + 4 cl 2 ⟶ ccl 4 + 4 hcl ccl 4 + 2 hf ⟶ ccl 2 f 2 + 2 hcl the ccl 4 formed in the first step is used as a reactant in the second step. if 3.00 mol ch 4 reacts, what is the total amount of hcl produced? assume that cl 2 and hf are present in excess.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 23:00
In the reaction h2co3 (aq) + 3nh3 (aq) = 2 nh4+ (aq) + co3 2-, how many electrons are transferred?
Answers: 3

Chemistry, 23.06.2019 02:00
Scientists are often interested in knowing the molar heat of combustion – the heat released during the combustion of one mole of a substance. use the periodic table to find molar masses. how many moles of ethanol are present in the sample?
Answers: 2

Chemistry, 23.06.2019 05:50
Which of the following is not a characteristic of s waves?
Answers: 1
You know the right answer?
Each step in the following process has a yield of 90.0 %. ch 4 + 4 cl 2 ⟶ ccl 4 + 4 hcl ccl 4 + 2 hf...
Questions

Mathematics, 20.12.2019 16:31



Social Studies, 20.12.2019 16:31

Mathematics, 20.12.2019 16:31

Biology, 20.12.2019 16:31

English, 20.12.2019 16:31

History, 20.12.2019 16:31

English, 20.12.2019 16:31




Mathematics, 20.12.2019 16:31



Mathematics, 20.12.2019 16:31

Mathematics, 20.12.2019 16:31

Biology, 20.12.2019 16:31


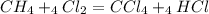
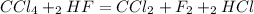
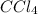 is formed in the first reaction.
is formed in the first reaction.