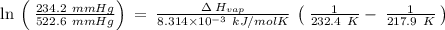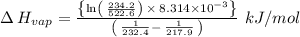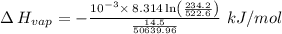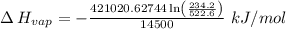Chemistry, 06.12.2019 00:31 graceaowen
The vapor pressure of the liquid nh3 is measured at different temperatures. the following vapor pressure data are obtained.
calculate the enthalpy of vaporization (? hvap) in kj/mol for this liquid.
p1 = 234.2 mmhg t1 = 217.9 k
p2 = 522.6 mmhg t2 = 232.4 k

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:50
The density of glycerin is 1.26grams/centimeter cubed . how many is this? use the conversion rates of and . express your answer to the correct number of significant figures.
Answers: 1

Chemistry, 21.06.2019 23:00
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1

Chemistry, 22.06.2019 12:20
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1

Chemistry, 23.06.2019 02:40
How can a mixture of salt water be separated into salt and water
Answers: 1
You know the right answer?
The vapor pressure of the liquid nh3 is measured at different temperatures. the following vapor pres...
Questions












Mathematics, 05.03.2020 05:11

Health, 05.03.2020 05:11






Mathematics, 05.03.2020 05:12

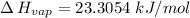
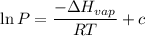
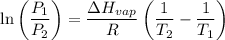
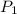 = 234.2 mmHg
= 234.2 mmHg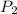 = 522.6 mmHg
= 522.6 mmHg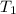 = 217.9 K
= 217.9 K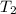 = 232.4 K
= 232.4 K