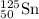Chemistry, 06.12.2019 00:31 ninilizovtskt
An atom of 125sn has a mass of 124.907785 amu. calculate the mass defect (deficit) in amu/atom. use the masses: mass of 1h atom = 1.007825 amu mass of a neutron = 1.008665 amu 1 amu = 931.5 mev give your answer to 4 significant figures and do not use e notation..

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 13:40
Can someone me with 6 to 10 plz this is for masteries test.
Answers: 1

Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1

Chemistry, 22.06.2019 22:00
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1
You know the right answer?
An atom of 125sn has a mass of 124.907785 amu. calculate the mass defect (deficit) in amu/atom. use...
Questions


Mathematics, 13.05.2021 20:40



Physics, 13.05.2021 20:40









Computers and Technology, 13.05.2021 20:40



Spanish, 13.05.2021 20:40

History, 13.05.2021 20:40