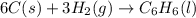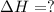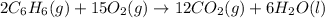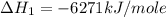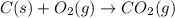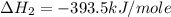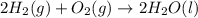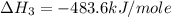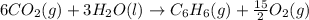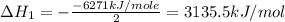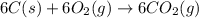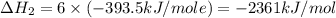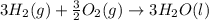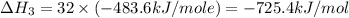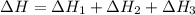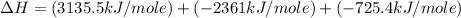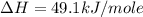Chemistry, 05.12.2019 23:31 carlinryan
Using the equations 2 c₆h₆ (l) + 15 o₂ (g) → 12 co₂ (g) + 6 h₂o (g)∆h° = -6271 kj/mol c (s) + o₂ (g) → co₂ (g) ∆h° = -393.5 kj/mol 2 h₂ (g) + o₂ (g) → 2 h₂o (g) ∆h° = -483.6 kj/mol determine the enthalpy for the reaction 6 c (s) + 3 h₂ (g) → c₆h₆ (l).

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:10
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3

Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 21:30
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3
You know the right answer?
Using the equations 2 c₆h₆ (l) + 15 o₂ (g) → 12 co₂ (g) + 6 h₂o (g)∆h° = -6271 kj/mol c (s) + o₂ (g)...
Questions

Mathematics, 18.06.2021 15:00


History, 18.06.2021 15:00


Business, 18.06.2021 15:00

Computers and Technology, 18.06.2021 15:00


Computers and Technology, 18.06.2021 15:10

Chemistry, 18.06.2021 15:10



Mathematics, 18.06.2021 15:10

Chemistry, 18.06.2021 15:10

Mathematics, 18.06.2021 15:10


Mathematics, 18.06.2021 15:10


Mathematics, 18.06.2021 15:10

Mathematics, 18.06.2021 15:10

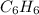 will be,
will be,