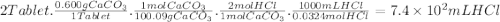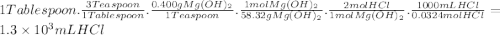The daily output of stomach acid (gastric juice) is 1000 to 2000 ml. prior to a meal, stomach acid (hcl) typically has a ph of 1.49.b.) one chewable tablet of the antacid maalox contains 600. mg of caco3. enter the neutralization equation. express your answer as a molecular equation including phasesc.) given that one chewable tablet of the antacid maalox contains 600. mg of caco3, calculate the milliliters of stomach acid neutralized by two tablets of maalox. express the volume in milliliters to two significant figures. d.) the antacid milk of magnesia contains 400. mg of mg(oh)2 per teaspoon. enter the neutralization equation. express your answer as a molecular equation including phases. e.) given that the antacid milk of magnesia contains 400. mg of mg(oh)2 per teaspoon, calculate the number of milliliters of stomach acid that are neutralized by 1 tablespoon of milk of magnesia. (1 tablespoon = 3 teaspoons.)express the volume in milliliters to two significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

You know the right answer?
The daily output of stomach acid (gastric juice) is 1000 to 2000 ml. prior to a meal, stomach acid (...
Questions