
Chemistry, 05.12.2019 22:31 cuthbertson157
Dissolving 5.28 g of an impure sample of calcium carbonate in hydrochloric acid produced 1.14 l of carbon dioxide at 20.0 â°c and 791 mmhg. calculate the percent by mass of calcium carbonate in the sample.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 21:30
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2

Chemistry, 23.06.2019 04:20
The equation below shows a chemical reaction. a + b + heat —> c + d according to the law of conservation of energy, which statement is true? a. the reactants absorb heat because they have less energy than the products. b. the products release heat because they have more energy than the reactants. c. the reactants generate heat because they have more energy than the products. d. the products require heat to form because they have less energy than the reactants.
Answers: 1

Chemistry, 23.06.2019 07:40
)in the deacon process for the manufacture of chlorine, hcl and o2 react to form cl2 and h2o. sufficient air (21 mole% o2, 79% n2) is fed to provide 35% excess oxygen, and the fractional conversion of hcl is 85%. calculate the mole fractions of the product stream components.
Answers: 1
You know the right answer?
Dissolving 5.28 g of an impure sample of calcium carbonate in hydrochloric acid produced 1.14 l of c...
Questions

Mathematics, 23.03.2021 17:20

Mathematics, 23.03.2021 17:20

Mathematics, 23.03.2021 17:20




English, 23.03.2021 17:20

Mathematics, 23.03.2021 17:20

Physics, 23.03.2021 17:20

History, 23.03.2021 17:20

Mathematics, 23.03.2021 17:20


Mathematics, 23.03.2021 17:20

Mathematics, 23.03.2021 17:20


Chemistry, 23.03.2021 17:20

Mathematics, 23.03.2021 17:20

Mathematics, 23.03.2021 17:20

Mathematics, 23.03.2021 17:20

Mathematics, 23.03.2021 17:20

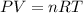



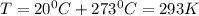



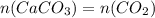

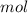 * 100
* 100 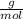 = 4.93839
= 4.93839 
 =
= 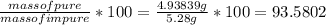 .
.

