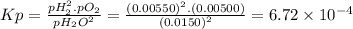Chemistry, 05.12.2019 22:31 jacxirylopez
The elementary reaction 2 h 2 o ( g ) − ⇀ ↽ − 2 h 2 ( g ) + o 2 ( g ) proceeds at a certain temperature until the partial pressures of h 2 o , h 2 , and o 2 reach 0.0150 bar , 0.00550 bar , and 0.00500 bar respectively. what is the value of the equilibrium constant at this temperature?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 01:30
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 08:00
Nconcentration refers to the molar concentration of an ion in solution. it may be identical to, or greater or less than, the molar concentration of the compound containing the ion that was used to make the solution. for soluble salts, the molarity of a particular ion is equal to the molarity of that compound times the subscript for that ion. for example, 1 m of alcl3 is 1 m in al3+ and 3 m in cl−. 1 m of (nh4)2so4 is 2 m in nh4+ and 1 m in so42−. part a what is the concentration of k+ in 0.15 m of k2s? view available hint(s) nothing m m part b if cacl2 is dissolved in water, what can be said about the concentration of the ca2+ ion? view available hint(s) if is dissolved in water, what can be said about the concentration of the ion? it has the same concentration as the cl− ion. its concentration is half that of the cl− ion. its concentration is twice that of the cl− ion. its concentration is one-third that of the cl− ion. part c a scientist wants to make a solution of tribasic sodium phosphate, na3po4, for a laboratory experiment. how many grams of na3po4 will be needed to produce 550 ml of a solution that has a concentration of na+ ions of 0.700 m ? express your answer numerically in grams. view available hint(s) mass of na3po4 n a 3 p o 4 = nothing g provide feedback
Answers: 3
You know the right answer?
The elementary reaction 2 h 2 o ( g ) − ⇀ ↽ − 2 h 2 ( g ) + o 2 ( g ) proceeds at a certain temperat...
Questions

Biology, 14.05.2021 08:30


Business, 14.05.2021 08:30

English, 14.05.2021 08:30



Mathematics, 14.05.2021 08:30


Mathematics, 14.05.2021 08:30


Mathematics, 14.05.2021 08:30

English, 14.05.2021 08:30

Physics, 14.05.2021 08:30


Mathematics, 14.05.2021 08:30


English, 14.05.2021 08:30

History, 14.05.2021 08:30

Mathematics, 14.05.2021 08:30