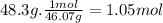Chemistry, 05.12.2019 21:31 rikac72791
How much heat energy is required to convert 48.3 g of solid ethanol at -114.5 degree c to gasesous ethanol at 135.3 degree c? the molar heat of fusion of ethanol is 4.60 kj/mol and its molar heat of vaporization is 38.56 kj/mol. ethanol has a normal melting point of -114.5 degree c and a normal boiling point of 78.4 degree c. the specific heat capacity of liquid ethanol is 2.45 j/g degree c and that of gaseous ethanol is 1.43 j/g degree

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Problem page gaseous ethane reacts with gaseous oxygen gas to produce gaseous carbon dioxide and gaseous water . if of water is produced from the reaction of of ethane and of oxygen gas, calculate the percent yield of water. be sure your answer has the correct number of significant digits in it.
Answers: 2


Chemistry, 22.06.2019 10:10
When electrolyzing copper (ll) chloride, what reaction takes place at the anode? what reaction takes place at the cathode?
Answers: 1

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1
You know the right answer?
How much heat energy is required to convert 48.3 g of solid ethanol at -114.5 degree c to gasesous e...
Questions


History, 20.02.2021 22:00

Biology, 20.02.2021 22:00





History, 20.02.2021 22:00

Advanced Placement (AP), 20.02.2021 22:00

Mathematics, 20.02.2021 22:00

Mathematics, 20.02.2021 22:00

Mathematics, 20.02.2021 22:00

Geography, 20.02.2021 22:00

History, 20.02.2021 22:00


Chemistry, 20.02.2021 22:00

Biology, 20.02.2021 22:00


Mathematics, 20.02.2021 22:00

Mathematics, 20.02.2021 22:00