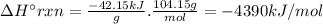Chemistry, 05.12.2019 20:31 leeamation31
Styrene, c8h8, is one of the substances used in the production of synthetic rubber. when styrene burns in oxygen to form carbon dioxide and liquid water under standard-state conditions at 25°c, 42.15 kj are released per gram of styrene. find the standard enthalpy of formation of styrene at 25°c.
(given: ? h°f[co2(g)] = –393.5 kj/mol, ? h°f[h2o(l)] = –285.8 kj/mol, ? h°f[h2o(g)] = –241.8 kj/mol)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Is this a scientific model? use complete sentences to explain why or why not. a graphic organizer showing the water cycle i need : ( asap i go it never mind
Answers: 2

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1
You know the right answer?
Styrene, c8h8, is one of the substances used in the production of synthetic rubber. when styrene bur...
Questions