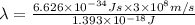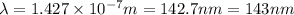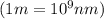Chemistry, 05.12.2019 20:31 arianawelsh123l
It takes 839./kjmol to break a carbon-carbon triple bond. calculate the maximum wavelength of light for which a carbon-carbon triple bond could be broken by absorbing a single photon.
round your answer to 3 significant digits in nm.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

Chemistry, 22.06.2019 07:30
Aradio signal from a gps satellite take only about 0.067 seconds to reach a gps reciever. if the speed of light is about 300,000km/s, then approximately how far away is the reciever from from the satellite?
Answers: 1

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2
You know the right answer?
It takes 839./kjmol to break a carbon-carbon triple bond. calculate the maximum wavelength of light...
Questions


Computers and Technology, 16.02.2021 06:20

Arts, 16.02.2021 06:20

Mathematics, 16.02.2021 06:20

Mathematics, 16.02.2021 06:20

French, 16.02.2021 06:20



English, 16.02.2021 06:20

Spanish, 16.02.2021 06:20

Health, 16.02.2021 06:20

Arts, 16.02.2021 06:20


Physics, 16.02.2021 06:20

Mathematics, 16.02.2021 06:20

English, 16.02.2021 06:20

Biology, 16.02.2021 06:20



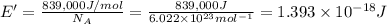
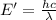 (Using planks equation)
(Using planks equation)