
To identify a diatomic gas (x2), a researcher carried out the following experiment: she weighed an empty 5.8-l bulb, then filled it with the gas at 1.00 atm and 21.0 ∘c and weighed it again. the difference in mass was 6.7 g. identify the gas. express your answer as a chemical formula.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2

Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2

Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2

Chemistry, 23.06.2019 01:30
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1
You know the right answer?
To identify a diatomic gas (x2), a researcher carried out the following experiment: she weighed an...
Questions

Mathematics, 21.04.2021 19:00

Mathematics, 21.04.2021 19:00




Mathematics, 21.04.2021 19:00

Health, 21.04.2021 19:00




Mathematics, 21.04.2021 19:00


Mathematics, 21.04.2021 19:00

Mathematics, 21.04.2021 19:00


English, 21.04.2021 19:00

Mathematics, 21.04.2021 19:00

Mathematics, 21.04.2021 19:00


Mathematics, 21.04.2021 19:00

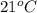 = (21 + 273) K = 294 K, mass = 6.7 g
= (21 + 273) K = 294 K, mass = 6.7 g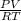
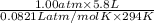
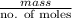
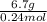
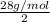
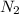 .
.

