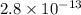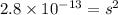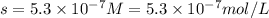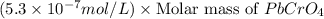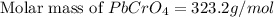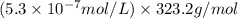Chemistry, 05.12.2019 18:31 BrainlyAvenger
G[mcquarrie 22-7] the value of ksp for pbcro4(s) in equilibrium with water at 25◦c is 2.8 · 10−13m2 . write the chemical equation that represents the solubility equilibrium for pbcro4(s) and calculate its solubility in grams per liter in water at 25◦c.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
150.0 grams of asf3 were reacted with 180.0 g of ccl4 to produce ascl3 and ccl2f2. if the actual yield of ccl2f2 was 127 g, what is the percent yield?
Answers: 2

Chemistry, 22.06.2019 01:40
Darla claims that the first periodic table developed by mendeleev was not completely accurate, so it is not useful at all. harmony argues that it establish the periodic table we use today, making it more credible. who is correct and why? darla is correct, because a model that has any mistakes should be thrown out. darla is correct, because a good model would not need to change. harmony is correct, because mendeleev’s model had all of the information correct in the first version. harmony is correct, because mendeleev’s model made predictions that came true.
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2
You know the right answer?
G[mcquarrie 22-7] the value of ksp for pbcro4(s) in equilibrium with water at 25◦c is 2.8 · 10−13m2...
Questions

Mathematics, 26.09.2019 04:50

Mathematics, 26.09.2019 04:50


History, 26.09.2019 04:50

Mathematics, 26.09.2019 04:50

Physics, 26.09.2019 04:50


History, 26.09.2019 04:50



Mathematics, 26.09.2019 04:50





Mathematics, 26.09.2019 04:50

Biology, 26.09.2019 04:50

Mathematics, 26.09.2019 04:50


Mathematics, 26.09.2019 04:50

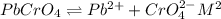
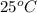 is,
is, 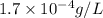
![K_{sp}=[Pb^{2+}][CrO_4^{2-}]](/tpl/images/0404/8064/04f28.png)
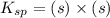
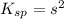
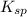 =
=