
Chemistry, 05.12.2019 05:31 Jcausey4477
A) how many moles of co2 and h2o are formed from 3.85 mole of propane c3h8 (this calculation needs to be done twice-once fro co2 and once for h20.
b) if 0.647 mole of oxygen in used in the burning of propane, how many moles of each of h2o are produced? how many moles of c3h8 are consumed?
the balanced chemical reaction:
1 c3 + 5 o2 = 3co2 + 4 h2o

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2


Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1

Chemistry, 22.06.2019 23:00
What is the number of neutrons in an atom with atomic mass of 35
Answers: 2
You know the right answer?
A) how many moles of co2 and h2o are formed from 3.85 mole of propane c3h8 (this calculation needs t...
Questions

Biology, 10.09.2020 23:01

Mathematics, 10.09.2020 23:01

English, 10.09.2020 23:01

Mathematics, 10.09.2020 23:01

World Languages, 10.09.2020 23:01

Mathematics, 10.09.2020 23:01

History, 10.09.2020 23:01

Biology, 10.09.2020 23:01

Social Studies, 10.09.2020 23:01

Physics, 10.09.2020 23:01

Mathematics, 10.09.2020 23:01

English, 10.09.2020 23:01

Mathematics, 10.09.2020 23:01

Mathematics, 10.09.2020 23:01

Mathematics, 10.09.2020 23:01

Mathematics, 10.09.2020 23:01

Mathematics, 10.09.2020 23:01

Mathematics, 10.09.2020 23:01

Mathematics, 10.09.2020 23:01

Mathematics, 11.09.2020 01:01

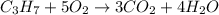
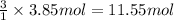 of carbon dioxide gas.
of carbon dioxide gas.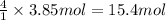 of water .
of water .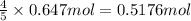 of water.
of water.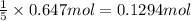 of propane.
of propane.

