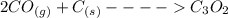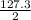2co (g) + c (g) -> c3o2 (g)
delta h = 127.3 kj/mol (rxn)
the equation above represen...


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2

Chemistry, 22.06.2019 20:00
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3
You know the right answer?
Questions

Mathematics, 03.08.2019 04:30


Mathematics, 03.08.2019 04:30




Social Studies, 03.08.2019 04:30


Computers and Technology, 03.08.2019 04:30

Computers and Technology, 03.08.2019 04:30



Mathematics, 03.08.2019 04:30



Mathematics, 03.08.2019 04:30