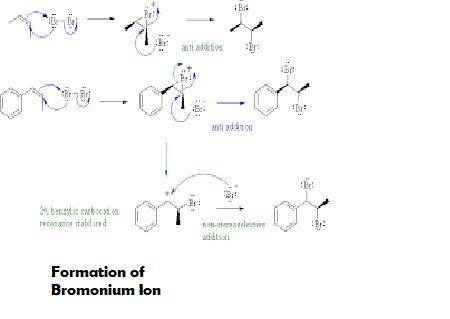
Two different bromonium ions are formed because br2 can add to the double bond either from the top of the plane or from the bottomtwo different bromonium ions are formed because {\rm br_2} can add to the double bond blank of the plane defined by the double bond, and the two bromonium ions are formed in equal amounts. of the plane defined by the double bond, and the two bromonium ions are formed in equal amounts.
a) true
b) false

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2


Chemistry, 23.06.2019 11:00
Acompound is isolated from the rind of lemons that is found to be 88.14% carbon and 11.86% hydrogen by mass how many grams of c and h?
Answers: 2
You know the right answer?
Two different bromonium ions are formed because br2 can add to the double bond either from the top o...
Questions


Mathematics, 16.10.2020 08:01

Social Studies, 16.10.2020 08:01

Mathematics, 16.10.2020 08:01

English, 16.10.2020 08:01

History, 16.10.2020 08:01

English, 16.10.2020 08:01

Mathematics, 16.10.2020 08:01

English, 16.10.2020 08:01



Social Studies, 16.10.2020 08:01





Arts, 16.10.2020 08:01



History, 16.10.2020 08:01