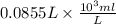Chemistry, 04.12.2019 21:31 magicalforlife
You have 0.500 l of an 0.250 m acetate buffer solution (i. e. [hc₂h₃o₂] + [c₂h₃o₂⁻] = 0.250 m) at ph 3.50. how many ml of 1.000 m naoh must you add in order to change the ph to 5.25? acetic acid has a pka of 4.74.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:50
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3


Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2
You know the right answer?
You have 0.500 l of an 0.250 m acetate buffer solution (i. e. [hc₂h₃o₂] + [c₂h₃o₂⁻] = 0.250 m) at ph...
Questions

Mathematics, 04.02.2021 06:30

Mathematics, 04.02.2021 06:30

Mathematics, 04.02.2021 06:30

English, 04.02.2021 06:30

Engineering, 04.02.2021 06:30

Engineering, 04.02.2021 06:30

Mathematics, 04.02.2021 06:30

Chemistry, 04.02.2021 06:30

Chemistry, 04.02.2021 06:30

Chemistry, 04.02.2021 06:30



Chemistry, 04.02.2021 06:30



Social Studies, 04.02.2021 06:30


English, 04.02.2021 06:30

Mathematics, 04.02.2021 06:30

Mathematics, 04.02.2021 06:30

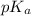 is as follows.
is as follows.![pK_{a} + log \frac{[CH_{3}COO^{-}]}{[CH_{3}COOH]}](/tpl/images/0403/2718/85431.png)
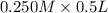
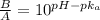
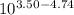
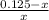 = 0.0575
= 0.0575![log \frac{[B]}{[A]}](/tpl/images/0403/2718/93cf8.png)
![log \frac{[B]}{[A]} = 10^{5.25 - 4.74}](/tpl/images/0403/2718/76315.png)
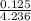
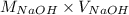 = (0.118 - 0.0295) moles
= (0.118 - 0.0295) moles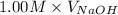 = 0.0885 moles
= 0.0885 moles