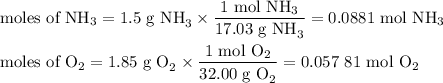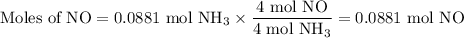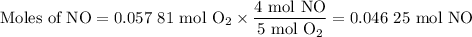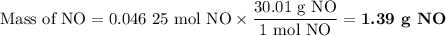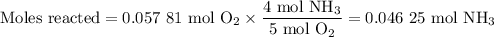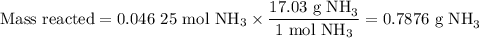no:

Chemistry, 04.12.2019 19:31 ErickP1686
1.) the process for converting ammonia to nitric acid involves the conversion of nh3 to
no:
nh3 + o2 + no + h2o
balanced: 4nh3 +502 uno+ 6h2o
a.) how many grams of no form when 1.5g of nh3 reacts with 1.85g of o2? b.) which
reactant is the limiting reactant and which one is the excess reactant? c.) how much of
the excess reactant remains after the limiting reactant is completely consumed?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1



You know the right answer?
1.) the process for converting ammonia to nitric acid involves the conversion of nh3 to
no:
no:
Questions

Mathematics, 12.03.2021 01:20

History, 12.03.2021 01:20


Mathematics, 12.03.2021 01:20

Spanish, 12.03.2021 01:20

SAT, 12.03.2021 01:20

Physics, 12.03.2021 01:20

Mathematics, 12.03.2021 01:20

Arts, 12.03.2021 01:20

Law, 12.03.2021 01:20

Mathematics, 12.03.2021 01:20


English, 12.03.2021 01:20

Mathematics, 12.03.2021 01:20

Mathematics, 12.03.2021 01:20


Physics, 12.03.2021 01:20


Social Studies, 12.03.2021 01:20

History, 12.03.2021 01:20