
The table below shows the results of an experiment done by juan and cindy. juan and cindy placed hydrogen peroxide on several food samples and recorded the relative amount of fizz that each produced. the fizz was produced by oxygen bubbles released by the action of the enzyme catalase, which is found in almost every living cell. which of these is a valid conclusion from their data?
a) plant cells produce more oxygen bubbles than animal cells.
b) boiled potato and cooked liver produced the most oxygen bubbles.
c) raw liver produced the most oxygen bubbles, followed by raw potato.
d) cooking the foods seems to increase the activity of the catalase enzyme.
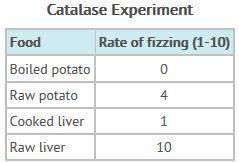

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1

Chemistry, 22.06.2019 19:40
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1

Chemistry, 23.06.2019 06:40
A250 g sample of water with an initial temperatureof 98.8 closes 6500 joules of heat. what is the finaltemperature of the water?
Answers: 1
You know the right answer?
The table below shows the results of an experiment done by juan and cindy. juan and cindy placed hyd...
Questions

History, 27.12.2021 02:50

Biology, 27.12.2021 02:50



Arts, 27.12.2021 03:00

Biology, 27.12.2021 03:00


History, 27.12.2021 03:00


Biology, 27.12.2021 03:00

Biology, 27.12.2021 03:00




Mathematics, 27.12.2021 03:10







