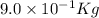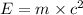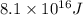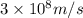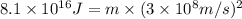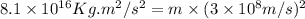Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1
You know the right answer?
In a nuclear reaction, the energy released is equal to 8.1 x 1016 joules. calculate the mass lost in...
Questions


English, 06.10.2019 09:00



English, 06.10.2019 09:00




Mathematics, 06.10.2019 09:00






Biology, 06.10.2019 09:00

Mathematics, 06.10.2019 09:00

Mathematics, 06.10.2019 09:00


Mathematics, 06.10.2019 09:00

Mathematics, 06.10.2019 09:00