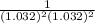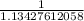Below regarding an electrochemical cell in an automotive lead-acid battery. the cell's anode is made of lead and the cathode is made of lead(iv) oxide. both are submerged in 4.30 m sulfuric acid. the half-reactions are: pbo_2(s) + 3h^+ (aq) + hso_4^- (aq) + 2e^- rightarrow pbso_4(s) + 2h_2o(l) e degree = 1.685 v pbso_4(s) + h^+(aq) + 2e^- rightarrow pb(s) + hso_4^- (aq) e degree = -0.356 v (a) calculate the value of e degree. (b) determine the initial value of e_cell. assume that the first ionization of h_2so_4 is complete and that [h^+] almostequalto [hso_4^-]. (c) find e_cell when the h^+ concentration has dropped by 76.00%. again, assume [h^+] almostequalto [hso_4^-].

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3

Chemistry, 23.06.2019 02:20
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2
You know the right answer?
Below regarding an electrochemical cell in an automotive lead-acid battery. the cell's anode is made...
Questions








Geography, 20.09.2020 04:01

Mathematics, 20.09.2020 04:01




Geography, 20.09.2020 04:01

Chemistry, 20.09.2020 04:01



Mathematics, 20.09.2020 04:01


Mathematics, 20.09.2020 04:01

Mathematics, 20.09.2020 04:01

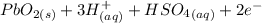 →
→ 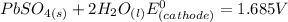
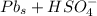 →
→ 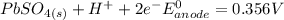
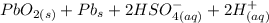 →
→ 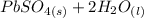
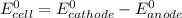
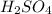 →
→ 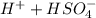
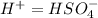 such that (;)
such that (;) 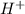 & b=
& b= 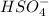
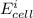 =
= 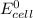 -
- 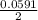 × log
× log 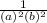
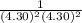
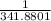
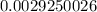
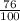
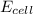 = 2.041V -
= 2.041V -