
Chemistry, 04.12.2019 04:31 tra10money
Classify each statement as true or false. drag the appropriate items to their respective bins. view available hint(s) reset the valence electrons of group 4a elements are in the 5s subshell. period 3 elements have an inner electron configuration of [ar). the highest principal quantum number of period 4 elements is 4 period 5 elements have six 4p electrons period 5 elements have an inner electron configuration of (kr group 8a elements have full outer principal s and p subshells. the valence electrons of group 3a elements are in an s and p subshell. the highest principal quantum number of period 4 elements is 5 false true submit

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Solar energy is energy from the sun that is converted into thermal or energy. a. nuclear b. mechanical c. electrical d. chemical
Answers: 2


Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

You know the right answer?
Classify each statement as true or false. drag the appropriate items to their respective bins. view...
Questions

English, 25.10.2019 06:43

English, 25.10.2019 06:43






Mathematics, 25.10.2019 06:43


History, 25.10.2019 06:43

History, 25.10.2019 06:43

History, 25.10.2019 06:43


Mathematics, 25.10.2019 06:43

Social Studies, 25.10.2019 06:43

Physics, 25.10.2019 06:43

English, 25.10.2019 06:43

Physics, 25.10.2019 06:43


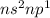 .
.

