
Chemistry, 04.12.2019 03:31 YoungManAlex
The value of kw at several different temperatures in given in the table below. what conclusion can be drawn on the basis of this information?
temperature
0ºc
25ºc
45ºc
kw
1.14 x 10-15
1.00 x 10-14
5.48 x 10-14
pure water becomes more alkaline as the temperature is increased.
the ionization of water is an exothermic process.
pure water becomes more acidic as the temperature is increased.
the ph of pure water decreases as the temperature is increased.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3

You know the right answer?
The value of kw at several different temperatures in given in the table below. what conclusion can b...
Questions

English, 24.11.2021 09:50

English, 24.11.2021 09:50

Mathematics, 24.11.2021 09:50

Computers and Technology, 24.11.2021 09:50


Mathematics, 24.11.2021 09:50



Mathematics, 24.11.2021 09:50


Health, 24.11.2021 09:50

History, 24.11.2021 09:50

Mathematics, 24.11.2021 09:50







Mathematics, 24.11.2021 09:50

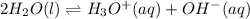
![K_w=[H_3O^+]\times [OH^-]](/tpl/images/0402/1575/b37c0.png)
![pH=-\log [H_3O^+]](/tpl/images/0402/1575/841e8.png)


