Chemistry, 04.12.2019 03:31 christophercordero15
Two nonpolar organic liquids, hexane (c6h14) and heptane (c7h16), are mixed. do you expect δhsoln to be a large positive number, a large negative number, or close to zero? fill in the following sentence: δhsoln is determined by the relative magnitudes of the "old" solute-solute (δhsolute) and solvent-solvent (δhsolvent) interactions and the "new" solute-solvent (δhmix) interactions. in this process, the energy of mixing hexane and heptane (δhmix) is the energy of separating them individually (δhsolute + δhsolvent), so δhsoln is expected to be .

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 02:10
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 22.06.2019 08:30
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2
You know the right answer?
Two nonpolar organic liquids, hexane (c6h14) and heptane (c7h16), are mixed. do you expect δhsoln to...
Questions




Mathematics, 06.01.2021 22:40

Mathematics, 06.01.2021 22:40



English, 06.01.2021 22:40

Mathematics, 06.01.2021 22:40

Mathematics, 06.01.2021 22:40

Mathematics, 06.01.2021 22:40



Mathematics, 06.01.2021 22:40


History, 06.01.2021 22:40


Mathematics, 06.01.2021 22:40


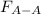 =
= 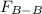 =
=