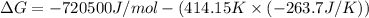Chemistry, 04.12.2019 03:31 ashcookie27
The value of delta g at 141.0 degrees celsius for the formation of phosphorous trichloride from its constituent elements,
p2(g) + 3cl2(g) > 2pcl3(g)
is kj/mol. at 25.0 degrees celsius for this reaction, delta h is -720.5 kj/mol, delta g is -642.9 kj/mol, and delta s is -263.7 j/k.
a.) -829.7
b.) 1.08 x 10^5
c.) 3.65 x 10^4
d.) -683.3
e.) -611.3

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:20
An aqueous solution of calcium hydroxide is standardized by titration with a 0.120 m solution of hydrobromic acid. if 16.5 ml of base are required to neutralize 27.5 ml of the acid, what is the molarity of the calcium hydroxide solution?
Answers: 3

Chemistry, 21.06.2019 15:00
Which element in the third period would you expect to have the larger atomic radius, sodium (na) or sulfur (s)? a. sodium, because it has a higher effective nuclear charge attracting electrons in fewer energy levels. b. sodium, because it has fewer protons attracting electrons in the same energy levels. c. sulfur, because it has more protons attracting electrons in more energy levels. d. sulfur, because it has a higher effective nuclear charge attracting electrons in the same energy levels.
Answers: 2

Chemistry, 21.06.2019 23:50
How does a scientist the government? a. the scientist tells people in society what to do. b. the scientist determines the policies that the government spends money on. c. the scientist provides unbiased information to the government. d. the scientist makes laws based on his or her research results.
Answers: 1

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2
You know the right answer?
The value of delta g at 141.0 degrees celsius for the formation of phosphorous trichloride from its...
Questions


Biology, 23.08.2019 05:10



Mathematics, 23.08.2019 05:10

Mathematics, 23.08.2019 05:10




Mathematics, 23.08.2019 05:10



English, 23.08.2019 05:10




Computers and Technology, 23.08.2019 05:10


Computers and Technology, 23.08.2019 05:10

Computers and Technology, 23.08.2019 05:10