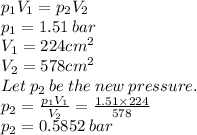6.
a gas occupies a volume of 224 cm and a pressure of 1.51 bars. if the gas expands to a volu...


Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2

Chemistry, 22.06.2019 17:20
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2
You know the right answer?
Questions

Mathematics, 02.09.2021 22:40

Mathematics, 02.09.2021 22:40




Mathematics, 02.09.2021 22:40


Mathematics, 02.09.2021 22:40





Mathematics, 02.09.2021 22:40


Mathematics, 02.09.2021 22:40

Mathematics, 02.09.2021 22:40

Mathematics, 02.09.2021 22:40


Mathematics, 02.09.2021 22:40