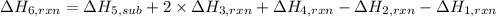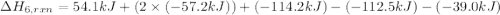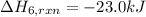Chemistry, 04.12.2019 02:31 austinparness02
The chemistry of nitrogen oxides is very versatile. given the following reactions and their standard enthalpy changes,
(1) no(g) + no
2
(g)
→
n
2
o
3
(g) ;
δ
h
o
r
x
n
= -39.kj
(2) no(g) + no
2
(g) + o
2
(g)
→
n
2
o
5
(g) ;
δ
h
o
r
x
n
= -112.5 kj
(3) 2no
2
(g)
→
n
2
o
4
(g) ;
δ
h
o
r
x
n
= -57.2 kj
(4) 2no(g) + o
2
(g)
→
2no
2
(g) ;
δ
h
o
r
x
n
= -114.2 kj
(5) n
2
o
5
(s)
→
n
2
o
5
(g) ;
δ
h
o
s
u
b
l
= 54.1 kj
calculate the heat of reaction for n
2
o
3
(g) + n
2
o
5
(s)
→
2n
2
o
4
(g)
δ
h = _ _ _ _ _ kj

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Si una estrella no tiene paralaje medible, ¿qué puedes inferir?
Answers: 1


Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1
You know the right answer?
The chemistry of nitrogen oxides is very versatile. given the following reactions and their standard...
Questions




Mathematics, 26.10.2021 14:00




English, 26.10.2021 14:00

Mathematics, 26.10.2021 14:00



Medicine, 26.10.2021 14:00

English, 26.10.2021 14:00

Mathematics, 26.10.2021 14:00


Mathematics, 26.10.2021 14:00

Physics, 26.10.2021 14:00


Computers and Technology, 26.10.2021 14:00

 ..[1]
..[1]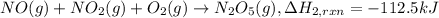 ..[2]
..[2]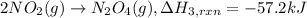 ..[3]
..[3]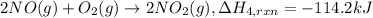 ..[4]
..[4]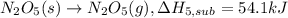 ..[5]
..[5]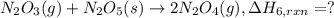 ..[6]
..[6]