
Chemistry, 04.12.2019 02:31 dancer2814
Line spectra from all regions of the electromagnetic spectrum, including the paschen series of infrared lines for hydrogen, are used by astronomers to identify elements present in the atmospheres of stars. calculate the wavelength of the photon emitted when the hydrogen atom undergoes a transition from n = 5 to n = 3. (r = 2.179 x 10-18 j r = 1.096776 x 10^7 m-1) a. 205.1 nm b. 384.6 nm c. 683.8 nm d. 1282 nm e. > 1500 nm

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 20:00
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1

Chemistry, 23.06.2019 01:00
What is the chemical name of the compound ti2o3? use the list of polyatomic ions and the periodic table to you answer.
Answers: 1

Chemistry, 23.06.2019 07:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1
You know the right answer?
Line spectra from all regions of the electromagnetic spectrum, including the paschen series of infra...
Questions


Chemistry, 01.07.2020 15:01


Physics, 01.07.2020 15:01


Mathematics, 01.07.2020 15:01






Physics, 01.07.2020 15:01








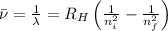
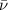 = Wave number
= Wave number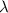 = Wavelength of radiation
= Wavelength of radiation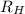 = Rydberg's Constant
= Rydberg's Constant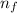 = Higher energy level
= Higher energy level 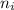 = Lower energy level
= Lower energy level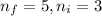
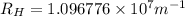
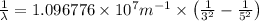
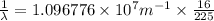
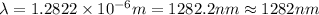
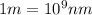 )
)

