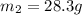Chemistry, 04.12.2019 02:31 kayleahrayne
Asample of nickel is heated to 99.8 degrees c and placed in a coffee cup calorimeter containing 150.0g water at 23.5 degrees c. after the metal cools, the final temperature of metal andwater mixture is 25.0 degrees c. if the specific heat capacity of nickel is 0.444j/(degreescg),
what mass of nickel was originally heated?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2
You know the right answer?
Asample of nickel is heated to 99.8 degrees c and placed in a coffee cup calorimeter containing 150....
Questions

Computers and Technology, 14.12.2021 05:30

Mathematics, 14.12.2021 05:30


Mathematics, 14.12.2021 05:30



Arts, 14.12.2021 05:30




Health, 14.12.2021 05:30

Mathematics, 14.12.2021 05:40


Spanish, 14.12.2021 05:40


Mathematics, 14.12.2021 05:40

English, 14.12.2021 05:40



Mathematics, 14.12.2021 05:40

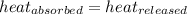
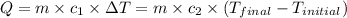
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0402/0900/09236.png) .................(1)
.................(1)
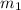 = mass of water= 150.0 g
= mass of water= 150.0 g
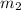 = mass of nickel = ?
= mass of nickel = ?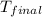 = final temperature =
= final temperature = 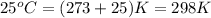
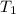 = temperature of water =
= temperature of water = 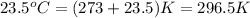
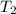 = temperature of nickel =
= temperature of nickel = 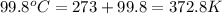
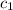 = specific heat of water =
= specific heat of water = 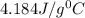
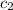 = specific heat of nickel=
= specific heat of nickel= 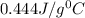
![150.0\times 4.184\times (298-296.5)=-[m_2\times 0.444\times (298-372.8)]](/tpl/images/0402/0900/54914.png)