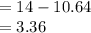Chemistry, 04.12.2019 02:31 jessnolonger
The acid-dissociation constants of hc3h5o3 and ch3nh3+ are given in the table below. which of the following mixtures is a buffer with a ph of approximately 3?
hc3h5o3
ch3nh3+
ka
8.3 x 10-4
2.3 x 10-11
a mixture of 100. ml of 0.1 m nac3h5o3 and 100. ml of naoh
a mixture of 100. ml of 0.1 m ch3nh3cl and 100. ml of ch3nh2
a mixture of 100. ml of 0.1 m hc3h5o3 and 50. ml of naoh
a mixture of 100. ml of 0.1 m ch3nh3cl and 50. ml of naoh

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3


Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1
You know the right answer?
The acid-dissociation constants of hc3h5o3 and ch3nh3+ are given in the table below. which of the fo...
Questions


Mathematics, 18.07.2019 13:00

Mathematics, 18.07.2019 13:00


Mathematics, 18.07.2019 13:00



Mathematics, 18.07.2019 13:00





Advanced Placement (AP), 18.07.2019 13:00

Health, 18.07.2019 13:00

Mathematics, 18.07.2019 13:00




French, 18.07.2019 13:00

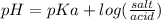
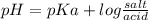
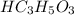 acid is closer to the required pH = 4 than
acid is closer to the required pH = 4 than 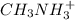 acid.
acid.
![pKa = -log [Ka]](/tpl/images/0402/1036/0b6c7.png)