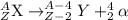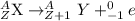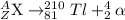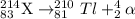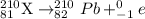Chemistry, 04.12.2019 02:31 MichaelG07
Bi undergoes four decay reactions: α, β, β, α. step 1. undergoes α decay to give in the second step, tl-210 undergoes β decay. what is the product of this step? symbol = mass number = atomic number =

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 23.06.2019 06:40
How many joules of heat are required to raise thetemperature of 750 g of water from 11.0 °c to 19.0 °c?
Answers: 1

Chemistry, 23.06.2019 15:30
How many moles of potassium nitrate, kno3 are present in a sample with a mass of 85.2 g?
Answers: 1

Chemistry, 23.06.2019 19:30
Is the following chemical equation balanced? agno3 + nacl 4agcl + nano3 yes no
Answers: 1
You know the right answer?
Bi undergoes four decay reactions: α, β, β, α. step 1. undergoes α decay to give in the second step...
Questions

Mathematics, 20.08.2019 13:30


Mathematics, 20.08.2019 13:30

Biology, 20.08.2019 13:30



History, 20.08.2019 13:30

Mathematics, 20.08.2019 13:30

Mathematics, 20.08.2019 13:30


Mathematics, 20.08.2019 13:30


Physics, 20.08.2019 13:30

Business, 20.08.2019 13:30

Mathematics, 20.08.2019 13:30



Mathematics, 20.08.2019 13:30


Biology, 20.08.2019 13:30